Intrinsically stretchable isoindigo–bithiophene conjugated copolymers using poly(acrylate amide) side chains for organic field-effect transistors†
Abstract
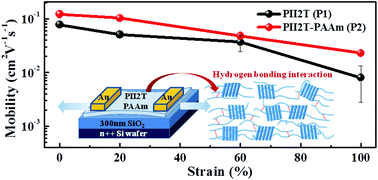
We report the synthesis, morphology, and properties of intrinsically stretchable isoindigo–bithiophene conjugated copolymers (PII2T) by incorporating octyldecane (OD) and poly(acrylate amide) (PAAm) side chains. Stronger molecular aggregation and crystallinity could be observed from the AFM morphology and GIXD analysis due to the hydrogen bonding interaction between the PAAm side chains. Thus, it achieved excellent stretchability and superior charge carrier mobility compared to the parent PII2T polymer. PAAm side chains were controlled with 5 (PAAm5) and 10 (PAAm10) repeating units, and the PII2T backbones were designed using 0, 5 and 10% PAAm side chains (P1–P3). The experimental results showed that PII2T with 5–10% PAAm5 were beneficial for the improvement of crystallinity and stretchability. However, PII2T with 5% PAAm10 (P4) exhibited poor crystallinity and charge carrier mobility due to the bulkiness of the side chains that disturb the molecular stacking. PII2T with 5% of the PAAm5 side chains showed a higher hole mobility of 0.30 cm2 V−1 s−1. Also, the soft segment in the PAAm side chains provided an amorphous domain facilitating stable mechanical properties. For PII2T with 5% of the PAAm5 chains, the cracking onset strain, the crystallinity preservation, and the elastic modulus for the transferred thin film increased from 20 to 60%, 37 to 61%, and 0.89 to 0.75 GPa, respectively, compared with the parent PII2T polymer. The double printed polymer thin films under an 80% strain had a stable hole mobility of 0.06 cm2 V−1 s−1. The improved stretchability and charge carrier mobility were attributed to the intrinsic softness of the PAAm side chains and the hydrogen bonding interaction. It suggests that the incorporation of hydrogen-bonded PAAm side chains into the PII2T backbone provides great potential for flexible and wearable electronic applications.


 Please wait while we load your content...
Please wait while we load your content...