Influence of an ester directing-group on defect formation in the synthesis of conjugated polymers via direct arylation polymerization (DArP) using sustainable solvents†
Abstract
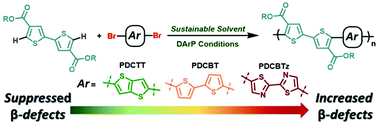
Direct arylation polymerization (DArP) is a synthetic methodology that allows for the preparation of conjugated polymers via C–H activation, facilitating a streamlined synthetic pathway for accessing monomers, while providing a reduction in the number of synthetic steps, hazardous waste, and toxic reagents. Improving the aspects of sustainability by changing the solvent or transition metal catalyst to a more sustainable alternative has recently garnered attention, and constitutes great importance for establishing DArP as an appealing alternative to commonly employed polymerization methods. Interestingly, while directing-groups are often employed for various small-molecule C–H couplings, use of these moeities have remained relatively unexplored despite their potential to enhance the reactivity of a given monomer. Towards these ends, we explore the use of the sustainable solvents cyclopentyl methyl ether (CPME) and anisole for the synthesis of a diester functionalized bithiophene using DArP to afford the copolymer poly[5,5′-bis(2-butyloctyl)-(2,2′-bithiophene)-4,4′-dicarboxylate-alt-5,5′-2,2′-bithiophene] (PDCBT) with a molecular weight (Mn) of 13.8 kDa and a yield of 59%. However, we observe the likely presence of branching (β) defects through analysis of 1H-NMR spectroscopy, GIXRD, and UV-vis absorption spectroscopy measurements. In order to determine if defect formation can be avoided, we study the occurance of defect formation as a function of the aryl spacer by employing the electron rich thieno[3,2-b]thiophene (PDCTT) and electron deficient 2,2′-bithiazole (PDCBTz). We find the optimal conditions provide PDCTT and PDCBTz with molecular weights of 26.4 and 4.9 kDa and yields of 90% and 46%, respectively, where PDCBTz was prone to form insoluble, branched polymer. This demonstrates that PDCBTz has a heightened reactivity towards defect formation, relative to PDCBT, while PDCTT does not, and indicates that such defect formation can be controlled through the appropriate selection of monomers. This study provides valuable insight regarding functional group tolerance and the capacity for esters to potentially function as directing-groups, leading to the undesired couplings of distal protons.


 Please wait while we load your content...
Please wait while we load your content...