Synthesis of well-defined glycopolymers with highly ordered sugar units in the side chain via combining CuAAC reaction and ROMP: lectin interaction study in homo- and hetero-glycopolymers†
Abstract
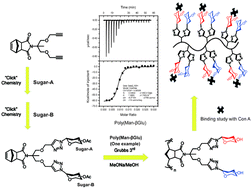
The heterogeneity of natural oligosaccharides containing various sugar units exists widely in molecular recognition, which can be utilized in enhancing the affinity and selectivity toward a specific receptor, thus the design of novel heterogeneous glycopolymers with different sugar motifs is of critical importance in the glycopolymer field. To investigate the significance of glycoheterogeneity in carbohydrate–protein binding, a series of well-defined homo-, hetero-glycopolymers and block, random glycopolymers with highly ordered sugar units in the side chain has been prepared by the combination of CuAAC reaction and ring-opening metathesis polymerization (ROMP). Isothermal titration calorimetry (ITC) measurements of the obtained glycopolymers with concanavalin A (Con A) as the model receptor indicated that the heteroglycopolymers, poly(Man-βGlu) and poly(Man-βGal) bearing both α-mannose and nonbinding β-glucose or β-galactose units, exhibit an approximately or more than 5-fold increase in their binding affinities to concanavalin A (Con A) as compared to monoglycopolymer poly(Man-Alkyne) and an over 1000-fold increase comparable to monosaccharide, methyl-α-mannose. The results revealed in this study further confirmed that heteroglycopolymers with non-binding sugar units present better specificity toward Con A than do monoglycopolymers with other functional groups in the side chain due to synergistic effects. As expected, both homoglycopolymers, poly(Man-Man) and poly(αGlu-αGlu) have strong binding activities towards Con A (Ka = 13.90 × 106 M−1, Ka = 9.52 × 106 M−1, respectively) due to multivalent binding and/or carbohydrate cluster effects. More intriguingly, the heteroglycopolymer, poly(Man-αGlu), exhibits near binding affinities to Con A in comparison with poly(Man-Man) supported by synergistic effects or an interplay where αGlu residues are added as weaker second binding units.


 Please wait while we load your content...
Please wait while we load your content...