PHEMA hydrogel films crosslinked with dynamic disulfide bonds: synthesis, swelling-induced mechanical instability and self-healing†
Abstract
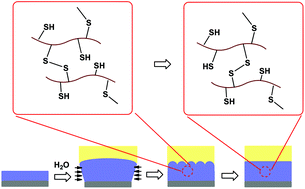
Swelling-induced mechanical instability is ubiquitous for substrate-attached hydrogel films, which results in a coarse surface and should be avoided in most applications. To test if the undesired instability patterns can be self-healed by the introduction of reversible covalent crosslinks, poly(2-hydroxyethyl methacrylate) (PHEMA) hydrogel films crosslinked with disulfide bonds were synthesized. 2-Acetylthioethyl methacrylate (ATEMA), a monomer containing a protected thiol group, was first synthesized from HEMA and thioacetic acid. Linear P(HEMA-ATEMA) copolymers were then synthesized by free-radical polymerization. To avoid the hydrolysis of ester bonds, the thioester protecting groups were cleaved by reaction with dithiothreitol in the presence of a catalytic amount of a base, and linear PHEMA polymers with pendant thiol groups, i.e., P(HEMA-MEMA) were obtained. PHEMA films crosslinked with disulfide bonds were obtained by exposing P(HEMA-MEMA) films to H2O2 vapor. Like ordinary hydrogel films, the crosslinked films also undergo mechanical instability when swelling in an ethanol/water mixture, because unidirectional expansion generates compressive stress within the film. Interestingly, the instability patterns could be self-healed, and the gel surface returned to be flat. In contrast, the instability patterns on a PHEMA film crosslinked with ordinary covalent bonds were permanent. The transient network in films crosslinked with dynamic disulfide bonds allows the relaxation of the swelling-induced compressive stress in the films, therefore, the undesired instability patterns can be self-healed.


 Please wait while we load your content...
Please wait while we load your content...