Synthesis of chain end acyl-functionalized polymers by living anionic polymerization: versatile precursors for H-shaped polymers†
Abstract
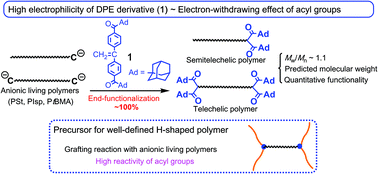
A series of α,ω-chain end acyl-functionalized polystyrenes with well-defined structures was synthesized by the 1 : 1 addition reaction between 1,1-bis(4-(1-adamantanecarbonyl)phenyl)ethylene (1) and difunctional living anionic polystyrenes prepared with lithium naphthalenide or potassium naphthalenide in THF at −78 °C after capping with 1,1-diphenylethylene (DPE). A tailored ω-functionalized polystyrene was similarly obtained by the reaction of 1 and DPE-capped living polystyrene initiated with sec-BuLi. The DPE-capped polyisoprenyl anion and poly(tert-butyl methacrylate) anion were also effective at preparing tailored end-functionalized polymers by the termination method using 1. The electrophilicity of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond in 1 was significantly enhanced by the electron-withdrawing effect of the 1-adamantylcarbonyl groups on the phenyl rings. The reaction of the living anionic polystyrene or poly(2-vinylpyridine) and the four C
C bond in 1 was significantly enhanced by the electron-withdrawing effect of the 1-adamantylcarbonyl groups on the phenyl rings. The reaction of the living anionic polystyrene or poly(2-vinylpyridine) and the four C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O terminal groups in the resulting end-functionalized polystyrene afforded H-shaped (co)polymers possessing well-defined structures. The introduced acyl terminal groups showed high reactivity toward nucleophiles, even in the presence of bulky and stiff adamantyl neighboring groups.
O terminal groups in the resulting end-functionalized polystyrene afforded H-shaped (co)polymers possessing well-defined structures. The introduced acyl terminal groups showed high reactivity toward nucleophiles, even in the presence of bulky and stiff adamantyl neighboring groups.


 Please wait while we load your content...
Please wait while we load your content...