Chiroptical phenolic resins grown on chiral silica-bonded amine residues†
Abstract
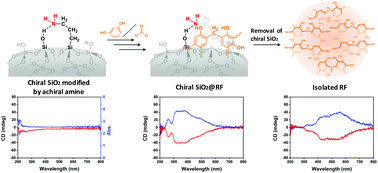
It has been generally recognized that chiral inorganic materials can be efficiently fabricated via a templated-synthesis route in the presence of chiral organic components of molecules or aggregates. However, there are few examples regarding the reverse process, that is, chirality transfer from chiral inorganics to other organics. Herein, we developed an asymmetric synthesis of chiral phenolic resins (RF) by employing synthetic chiral silica bonded covalently with amine residues as an asymmetric medium to asymmetrically mediate the polymerization of resorcinol (R) with formaldehyde (F). The resultant composites, consisting of RF resins and SiO2, showed chiroptical signals with a mirror relationship that appeared remarkably in their circular dichroism (CD) spectra around the adsorption bands of the RF resins. Interestingly, the RF resins still retained CD activities even after the removal of the chiral silica using HF (aq.), suggesting efficient chirality transfer from the chiral silica to the phenolic resins. We proposed that the origin of the chirality in the RF resins is the appearance of axial asymmetry due to the formation of distorted calixarene-like cyclic structures, which is supported by the emergence of chirality from the reaction of C-tetramethylcalix[4]resorcinarene and formaldehyde catalyzed by chiral silica-bonded amine residues.


 Please wait while we load your content...
Please wait while we load your content...