A kinetic study on the para-fluoro-thiol reaction in view of its use in materials design†
Abstract
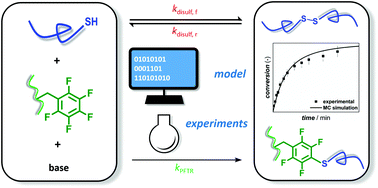
A detailed kinetic study on the para-fluoro-thiol reaction (PFTR) using experimental analysis and kinetic Monte Carlo modeling is introduced, covering the difference in reactivity of a selected variety of structurally different thiols, uniquely including polymeric thiols. The impact of solvent polarity is evaluated by comparing the results obtained using THF and DMF, with the latter giving the fastest reaction rates. As PFTR involves the formation of a thiolate ion, potential side reactions such as disulfide bond formation are studied both isolated (determination of disulfide rate coefficients) and in competition with PFTR (determination of PFTR rate coefficients and selectivities). It is demonstrated that for star polymer synthesis – employing a polymeric thiol and a trifunctional fluorinated linker – the PFTR reacitivity is influenced by diffusional limitations and that the disulfide formation needs to be considered. Different reaction conditions (e.g. addition of reducing agent) are explored to limit the disulfide bond formation, providing the user a guideline for optimizing PFTR upon application for macromolecular design at high reaction rate.


 Please wait while we load your content...
Please wait while we load your content...