Alicyclic tertiary amine based hyperbranched polymers with excitation-independent emission: structure, fluorescence and applications†
Abstract
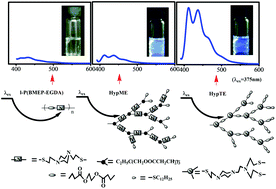
Amine-containing nonconventional luminogens without proverbial chromophores have attracted much attention recently. For the representative poly(amido amine) (PAMAM) system, influences of the tertiary amine structure and its position in the hyperbranched macromolecules on the fluorescence are still rarely discussed. In this paper, we prepared hyperbranched polymers only containing tertiary amines by linking alicyclic amines together. The comparisons of the inherent fluorescence emission among the linear polymers with piperazine in the backbone (l-P(BMEP-EGDA)) and as a side group (l-P(AMPMA-PDT)), the hyperbranched polymers with piperazine in the linear skeleton (HypMEs) and as branched units (HypTEs), and the hyperbranched polymers with acyclic tertiary amines as branched units (HypETs) illustrate that high fluorescence efficiency can be retained by fixing the tertiary amine in the branching units and decreasing the interior mobility of hyperbranched polymers. Due to the fixed emitting species, HypTEs showed excitation-independent emission. The galactopyranose-modified HypTE with low cytotoxicity and bright cell imaging can be potentially applied in biological fields.


 Please wait while we load your content...
Please wait while we load your content...