Synthesis of delayed-emissive poly(2,7-carbazole)s having an anchored triazine pendant at the N-position†
Abstract
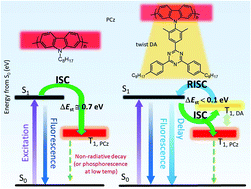
Several poly(2,7-carbazole)s having a planar triazine at the carbazole N-position were synthesized. Their torsion angles between the carbazole donor and the triazine acceptor units were controlled by phenylene connectors to separate their electronic functions. The polymers showed delayed fluorescence characteristics at room temperature besides phosphorescence with two different triplet energies at a liquid N2 temperature. The effect of π-conjugation along the polycarbazole main chain on the luminescence behavior was investigated by controlling the torsion angle between the carbazole units using substituents at 3,6-positions. The limitation of π-conjugation caused the overlapping of a phosphorescence spectrum with the fluorescence. They also showed solvent-dependent orientation properties like lyotropic liquid crystals. These new functions could be derived from the anchored acceptor unit in the polycarbazole.


 Please wait while we load your content...
Please wait while we load your content...