Self-catalysed folding of single chain nanoparticles (SCNPs) by NHC-mediated intramolecular benzoin condensation†
Abstract
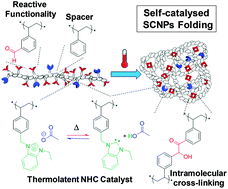
A self-catalysed folding strategy to form single chain nanoparticles (SCNPs) was developed via an intramolecular N-heterocyclic carbene (NHC)-mediated benzoin condensation. Benzaldehyde, styrene and benzimidazolium chloride units were randomly incorporated into a poly(ionic liquid)-based (PIL) copolymer precursor by reversible addition–fragmentation chain transfer (RAFT) copolymerisation. Post-chemical modification of this linear precursor by insertion of a non-innocent acetate counter-anion into the benzimidazolium moieties conferred thermolatent catalytic behaviour owing to the equilibrium between benzimidazolium acetate units and corresponding NHC ones. Upon heating, catalytically active NHCs allowed the formation of benzoin-type intramolecular cross-links, thus folding linear chains into SCNPs. NHC moieties became deactivated after cooling to room temperature, which enabled easy isolation and purification of the covalently and intramolecularly cross-linked SCNPs. Importantly, the latent NHC moieties were proven to retain their catalytic activity when arranged into SCNPs as evidenced through their reactivation by simple heating. The catalytic activity of these SCNPs was further confirmed by implementing another NHC-organocatalysed reaction, namely, transesterification reaction between vinyl acetate and benzyl alcohol. This work represents the first example of catalysis by a parent linear precursor which drives its own folding and remains catalytically active.
- This article is part of the themed collection: Polymer Chemistry Lectureship Winners


 Please wait while we load your content...
Please wait while we load your content...