Coumarines as masked phenols for amide functional benzoxazines†
Abstract
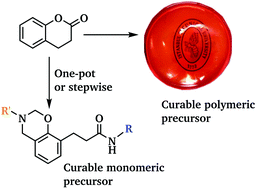
Benzoxazines with amide linkages were successfully prepared. Initially, 3,4-dihydrocoumarine (DHC) was used as the reagent to synthesize phenolic amides via a ring-opening reaction using primary amines. The phenolic amides were then reacted with formaldehyde and primary amines to produce amide containing benzoxazines. Moreover, the polymeric amide functional benzoxazine precursor was prepared with bisamine end-functional poly(propylene oxide) by Mannich type polycondensation. The obtained polymer exhibited good film forming properties and free standing flexible films were easily solvent-cast on glass plates. All of the monomers and polymeric precursors were characterized by 1H NMR and FTIR spectroscopic analysis and their curing behavior and thermal stability were investigated by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). Additionally, the mechanical properties of both cured and uncured polymeric benzoxazine precursors were studied by tensile test measurements.


 Please wait while we load your content...
Please wait while we load your content...