Tuning the dielectric behavior of poly(vinylidene fluoride-co-vinyl alcohol) using a facile urethane-based crosslinking method†
Abstract
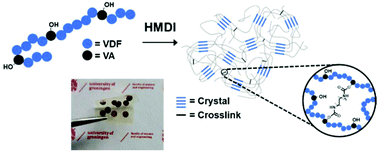
Crosslinking of polar fluorinated polymers, such as poly(vinylidene fluoride) (PVDF) and its copolymers, is receiving considerable research attention for capacitive energy storage applications due to their high polarization and large breakdown strength. Nowadays, methods to chemically crosslink PVDF-based materials to achieve higher energy densities with increased efficiencies are limited and often require external processing equipment. To overcome this problem, we demonstrate a new approach by introducing vinyl alcohol (VA) units in the PVDF backbone that can easily be crosslinked using urethane chemistry. The degree of crosslinking is systematically varied by using 0.5- (C05), 1.0- (C1) and 20-fold (C20) excess of isocyanate (hexamethylene diisocyanate) compared to hydroxyl groups. This has led to a reduction in average crystallite size from 12.1 nm for pristine poly(VDF-co-VA) to 6.4 nm and 6.1 nm for C05 and C1, respectively, while a 20-fold excess of isocyanate yielded an amorphous fluorinated network. The crystallinity reduced drastically from 38% for pristine poly(VDF-co-VA) to 13% (C05) and 5.4% (C1) upon higher degrees of crosslinking. The crystal lattice spacing of 4.30 Å remained unchanged for C05, while it slightly increased for C1 to 4.33 Å. Additionally, crosslinking reduced the melting temperature from 124 °C for poly(VDF-co-VA) to 91 °C (C05) and 86 °C (C1). The change in crystallite size and crystallinity has drastically influenced the interactions between the ferroelectric domains, leading to a change from ferroelectric to double hysteresis loop behavior. When an amorphous highly crosslinked network is obtained, the crosslinked copolymer behaves as a regular linear dielectric. In addition to the change in the electroactive behavior, the breakdown strength and reliability of the networks are significantly increased after crosslinking, which is reflected in the larger stored and discharged energy densities. Since the crosslinked samples show slimmer polarization due to a strong reduction in ferroelectric loss, higher charge–discharge efficiencies are observed. Therefore, this work demonstrates a simple solution processing method and straightforward urethane chemistry to crosslink poly(VDF-co-VA), showing high potential for the preparation of dielectric materials with high energy densities and improved efficiencies, while providing useful insights into the crystallization behavior for fine-tuning the PVDF crystals for further development in this field.


 Please wait while we load your content...
Please wait while we load your content...