Towards bio-based tapered block copolymers: the behaviour of myrcene in the statistical anionic copolymerisation†
Abstract
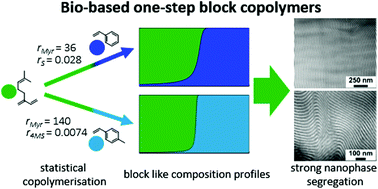
To explore the potential of myrcene (Myr) as a bio-based monoterpene comonomer for styrenic copolymers and to establish its general applicability for the carbanionic copolymerisation, several statistical copolymerisations of myrcene and common monomers like isoprene (I), styrene (S) and 4-methylstyrene (4MS) were carried out in cyclohexane and monitored by in situ1H NMR spectroscopy. Real-time NMR kinetic studies permitted the determination of the reactivity ratios and the composition profile for each monomer combination. While the copolymerisation of Myr/I yielded a gradient copolymer and reactivity ratios of moderate disparity (rMyr = 4.4; rI = 0.23), the statistical copolymerisation of Myr/S and Myr/4MS afforded block-like, tapered copolymers due to highly diverging reactivity ratios (rMyr = 36; rS = 0.028 and rMyr = 140; r4MS = 0.0074). Furthermore, a terpolymerisation of Myr/I/4MS was studied by real-time NMR kinetics, revealing an alteration of the composition profile of 4MS towards a more block-like structure. Based on the kinetic studies, a series of Myr/I/4MS terpolymers and Myr/S copolymers was prepared by statistical living anionic copolymerisation. All copolymers showed narrow molecular weight distributions (SEC) and two glass transition temperatures (Tg,1 = −51 to −62 °C; Tg,2 = 93 to 107 °C), suggesting phase segregation. TEM and SAXS measurements revealed highly ordered lamellar morphologies for all copolymers with long range correlation and confirmed the block-like structure and behaviour of Myr/S and Myr/4MS copolymers prepared by statistical carbanionic copolymerisation.


 Please wait while we load your content...
Please wait while we load your content...