Aqueous dynamic covalent assembly of molecular ladders and grids bearing boronate ester rungs†
Abstract
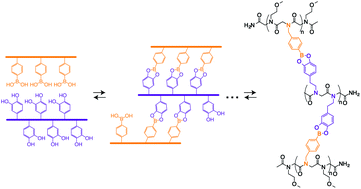
Mimicking the self-assembly of nucleic acid sequences into double-stranded molecular ladders that incorporate hydrogen bond-based rungs, dynamic covalent interactions enable the fabrication of molecular ladder and grid structures with covalent bond-based rungs. Here, we describe the synthesis of boronic acid- and catechol-bearing peptoid oligomers and utilize the dynamic, reversible condensation reaction between these reactive pendant groups to mediate the dynamic covalent assembly of complementary oligomers in aqueous solution, affording both molecular ladders and grids linked by covalent, boronate ester-based rungs. The generation of in-registry molecular ladders with up to six rungs and triplex molecular grids was confirmed by matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry, and the dynamic nature of the condensation reaction was demonstrated by rapid strand displacement with pre-assembled molecular ladders. Additionally, through the use of an indicator displacement assay with alizarin red S (ARS), the boronic acid/catechol binding constant for the formation of molecular ladders was determined.


 Please wait while we load your content...
Please wait while we load your content...