The fate of copper catalysts in atom transfer radical chemistry†
Abstract
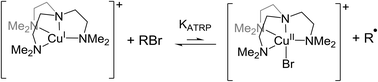
Recent experimental observations of the role played by copper polyamine catalysts in atom transfer radical polymerisation (ATRP) have indicated that important side reactions in competition with conventional radical activation by halogen atom transfer may occur and that these can potentially affect reaction control. Specifically the copper(I) catalyst may recapture the organic radical and even possibly abstract H-atoms from the radical to form copper(II) alkyl or hydrido complexes. Employing an established electrochemical methodology and supported by Density Functional Theory calculations, we report a systematic experimental and theoretical investigation of the ATRP catalyst [Cu(Me6tren)]+ (Me6tren = tris(2-dimethylaminoethyl)amine) and its reactions with a homologous series of bromo-ester initiators that yield tertiary, secondary and primary radicals. The results reveal that organocopper(II) complexes are preferred to hydrido complexes and that increasing steric bulk at the initiator steers the reaction pathway away from the organocopper complex and toward the desired ATRP product.


 Please wait while we load your content...
Please wait while we load your content...