A simple and versatile route to amphiphilic polymethacrylates: catalytic chain transfer polymerisation (CCTP) coupled with post-polymerisation modifications†
Abstract
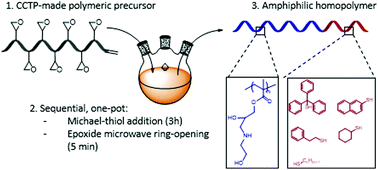
Amphiphilic polymers have become key figures in the fields of pharmacology, medicine, agriculture and cosmetics. The use of reversible deactivation radical polymerisation (RDRP) techniques has allowed advances in the synthesis of amphiphilic polymers. However, the high price to performance ratio of these methods can limit their industrial application. Herein, poly(glycidyl methacrylate) polymers of varying molecular weights were first synthesised by catalytic chain transfer polymerisation (CCTP). Amphiphilic polymers were then prepared using a simple one-pot, post-polymerisation modification process involving Michael-thiol addition in the presence of a range of hydrophobic mercaptans, followed by ring-opening of the epoxide groups with ethanolamine using microwave-assisted synthesis. This procedure allows for the synthesis of fully functional polymers within 3 hours. A range of well-defined materials are prepared and characterised by GPC, NMR, FTIR, DLS, TGA, and TEM.


 Please wait while we load your content...
Please wait while we load your content...