Enhanced reduction of polymerization-induced shrinkage stress via combination of radical ring opening and addition fragmentation chain transfer†
Abstract
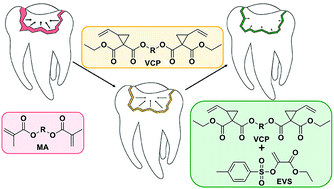
Polymerization-induced shrinkage stress within bulk photopolymer networks represents one of the most pressing challenges for their application in 3D-printing, microelectronics and dentistry. The occurring shrinkage stress within commercial (meth)acrylate-based networks results from a combination of the covalent attachment of monomers and the gelation at early stages of the reaction. Alternatively, cyclic monomers (e.g. 1,1-disubstituted 2-vinylcyclopropanes, VCPs) have been reported as a possible monomer class yielding reduced polymerization shrinkage and consequently shrinkage stress due to a radical ring opening reaction. Another way to reduce the occurring shrinkage stress is to regulate the radical network formation via an addition fragmentation chain transfer (AFCT) concept. Herein, the light-induced radical polymerization of a combination of VCP monomers with variable amounts of an AFCT reagent (an ester-activated vinyl sulfonate ester EVS) was investigated. A high reactivity towards light-induced radical polymerization is confirmed for VCP/EVS mixtures via photoreactor, photo-DSC and real time (RT)-NIR-photorheology accompanied by a significant increase in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond conversion with increasing amounts of EVS. Most importantly, both systems (VCPs and AFCT reagents) combined lead to an enhanced reduction of polymerization-induced shrinkage stress. The resulting materials showed, even at low concentrations of EVS, a high network homogeneity (indicated by a narrow loss factor plot in DMTA). Also, filled systems performed well with respect to reduced shrinkage force while maintaining sufficient E-modulus and flexural strength. The presented material concept has great potential for dental materials and lithography-based 3D-printing.
C double bond conversion with increasing amounts of EVS. Most importantly, both systems (VCPs and AFCT reagents) combined lead to an enhanced reduction of polymerization-induced shrinkage stress. The resulting materials showed, even at low concentrations of EVS, a high network homogeneity (indicated by a narrow loss factor plot in DMTA). Also, filled systems performed well with respect to reduced shrinkage force while maintaining sufficient E-modulus and flexural strength. The presented material concept has great potential for dental materials and lithography-based 3D-printing.


 Please wait while we load your content...
Please wait while we load your content...