One-pot cascade polymerization based on the addition reactions of electrophilic selenium reagents to alkenes†
Abstract
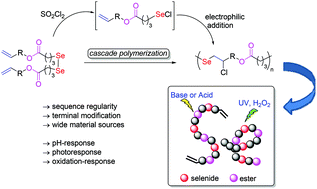
A direct polymerization based on the addition reactions of electrophilic selenium reagents to alkenes was established. It consists of a one-pot cascade process with the generation of selenyl chloride by in situ breakage of the Se–Se bond using sulfuryl chloride, followed by the addition of the obtained selenyl chloride to the carbon–carbon double bond. The polymerization mechanism was confirmed to be a stepwise mechanism. The resulting polymers were characterized and determined to be linear polymers with a sequence regulated backbone repeating unit of ester-selenide-vinyl chloride. When the proportion of sulfuryl chloride was controlled, vinyl terminal groups and diselenide could be easily introduced at the same time, providing a simple way for further modification and application. The feasibility of monomers derived from low cost and easily available unsaturated natural fatty alcohols such as oleyl alcohol, 5-norbornene-2-methanol, etc., was also investigated. It is worth emphasizing specially that the ester-selenide bridged backbone endowed the polyesters with multi stimuli-responsiveness for practical applications.
- This article is part of the themed collection: Selenium & Tellurium chemistry at the beginning of the 3rd millennium: a celebration of ICCST


 Please wait while we load your content...
Please wait while we load your content...