Topology effects on protein–polymer block copolymer self-assembly†
Abstract
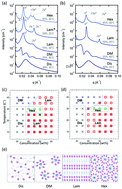
Bioconjugates made of the model red fluorescent protein mCherry and synthetic polymer blocks show that topology, i.e. the BA, BA2, ABA and ABC chain structure of the block copolymers, where B represents the protein and A and C represent polymers, has a significant effect on ordering transitions and the type and size of nanostructures formed during microphase separation. ABA and ABC type block copolymers were synthesized by using two site-specific bioconjugation reactions: the thiol–ene reaction with a cysteine on mCherry and maleimide functionalized polymers, and the sortase A ligation reaction with an LPETG sequence at the C-terminus on mCherry and a triglycine functionalized polymer. The phase behaviors of mCherry–poly(N-isopropylacrylamide) (PNIPAM) and mCherry–(PNIPAM)2 show that the shapes of the phase diagrams are similar overall, but mCherry–(PNIPAM)2, i.e. BA2 type, yields a narrower domain spacing than mCherry–PNIPAM, i.e. BA type. PNIPAM–mCherry–PNIPAM (ABA type) shows only lamellar phases in the range of conditions under which ordered structures appear. PDMAPS–mCherry–PNIPAM (ABC type) shows an ordered structure across the widest range of conditions in the four bioconjugates and also the widest range of different nanodomain structures. The phase behavior of the ABC type implies that the repulsive interaction between two water-soluble coil polymers can be a key factor in enhancing the self-assembly of globular protein–polymer block copolymers.


 Please wait while we load your content...
Please wait while we load your content...