Synthesis of highly reactive polyisobutylenes via cationic polymerization in ionic liquids: characteristics and mechanism†
Abstract
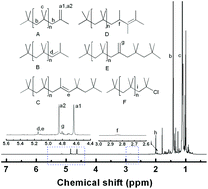
The cationic polymerization of isobutylene (IB) was systematically studied in a 1-butyl-3-methylimidazolium hexafluorophosphate ([Bmim][PF6]) ionic liquid at −10 °C. Different initiating systems, including titanium tetrachloride, boron trichloride, and ethylaluminum sesquichloride, were considered in [Bmim][PF6] for IB polymerization. The effects of solvent polarity and anion/cation structure on the initiating systems and carbocation active center were simulated by density functional theory in combination with a conductor-like screening model. A highly reactive polyisobutylene (HR PIB) with a high exo-olefin end group content (>80%) was synthesized by using a H2O/TiCl4 initiating system in the [Bmim][PF6] ionic liquid. The polymerization proceeded at the interface of ionic liquid particles in a mild exothermic manner and [PF6]− anions promoted the ionization of the initiating system and stabilized the carbocation active center. A possible mechanism for HR PIB synthesis was proposed and the microstructures of ionic liquids were considered.


 Please wait while we load your content...
Please wait while we load your content...