Functional polyamides with gem-diazido units: synthesis and diversification†
Abstract
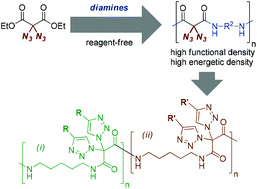
A diazidated malonate derivative was employed as a monomer for the reagent-free one-pot synthesis of several polyamides containing geminal diazido moieties within the polymer backbone. Using different diamines, azide-rich polyamides with diverse properties and sensitivities dependent on the structure of the diamine units were obtained. Postpolymerization CuAAC reactions of the geminal diazido units in the polymer backbone allowed for the introduction of additional functionalities, and the construction of densely functionalized polymeric structures.


 Please wait while we load your content...
Please wait while we load your content...