Ferrocene-containing amphiphilic polynorbornenes as biocompatible drug carriers†
Abstract
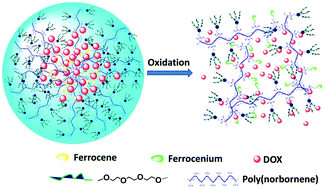
Redox-stimuli responsive amphiphilic copolymers have recently emerged as promising drug carrier systems with controllable drug release. Herein, we first reported the synthesis of two well-defined redox-stimuli responsive ferrocene-containing polymers, amphiphilic diblock copolymer PN(Fc-b-TEG) and random copolymer PN(Fc-r-TEG), via ring-opening metathesis polymerization (ROMP), and compared their properties as drug carriers. Results showed that both of the obtained copolymers could self-assemble into globular nanoscale core–shell micelles in aqueous solution and showed tunable redox responses. The copolymer micelles were used to entrap the hydrophobic dye Nile red (NR) or anticancer drug doxorubicin (DOX). The DOX encapsulation efficiency of PN(Fc-b-TEG) is 66.7% which is higher than that of PN(Fc-r-TEG) (21.1%) under the same conditions. Meanwhile, the DOX-loaded micelles exhibited oxidation-controlled drug release, and the release rate could be mediated by the concentration of oxidants. Cell counting kit (CCK) assay and model organism zebrafish embryo studies were further performed to disclose the biocompatibility and safety of the copolymer micelles, and the results showed that the copolymers had excellent biocompatibility. The neoteric ferrocene-containing copolymer carriers with reversible redox-response are anticipated to have potential in targeted drug delivery systems for cancer therapy.


 Please wait while we load your content...
Please wait while we load your content...