Photocatalysed decolouration of indigo in solution via in situ generation of an organic hydroperoxide†
Abstract
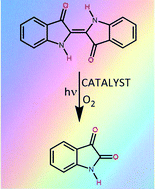
Indigo, an emblematic violet dye used for thousands of years to colour fabric, is resistant to fading on exposure to sunlight. Prior work has indicated that indigo is reactive towards both hydroperoxyl radicals and superoxide anions in solution. In order to promote photobleaching of indigo, we have utilised a BOPHY-based (BOPHY = aryl fused symmetrical pyrrole-BF2 complex) chromophore known to form both superoxide ions and a stable alkyl hydroperoxide under illumination in aerated solution. Selective irradiation of the photocatalyst causes relatively fast fading of indigo, with the rate increasing gently with increasing concentration of indigo. Molecular oxygen and light are essential for effective bleaching. One molecule of photocatalyst can bleach more than 40 molecules of indigo. An active component of the photocatalyst is a butylated hydroxytoluene (BHT) residue which itself quenches the triplet excited state of indigo. This provides an ancillary mechanism for effecting photofading of indigo but, because the triplet is formed in very low yield, this route is less practical.


 Please wait while we load your content...
Please wait while we load your content...