Design and characterization of a 2-(2′-hydroxyphenyl)benzimidazole-based Sr2+-selective fluorescent probe in organic and micellar solution systems†
Abstract
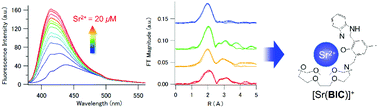
A novel Sr2+ fluorescent probe, N-(2-hydroxy-3-(1H-benzimidazol-2-yl)-phenyl)-1-aza-18-crown-6-ether (BIC), was synthesized and its fluorescence properties, equilibria, and local structure in solution were studied in detail. The fluorescence intensity of BIC in DMSO was enhanced selectively upon addition of Sr2+ but not Na+, K+, Mg2+, Ca2+, and Ba2+. To employ this rather hydrophobic BIC probe in aqueous media, a sodium laurate (LaNa) micellar solution was used as a good solvent. The detection limit of said LaNa micelle-BIC system (2.02 μM) is lower than that of the H2O system (309 μM), but higher than that of the DMSO system (0.04 μM). Therefore, it is clear that the LaNa micelles have an effect on the detection of Sr2+ by BIC in aqueous solutions. Further structural studies by extended X-ray absorption fine structure and speciation analyses revealed that BIC undergoes complexation equilibria corresponding to the formation of [Sr(BIC)]+ species in all solution types. It was concluded that the changes in the Sr2+-BIC fluorescence in solution are attributed to the formation of such [Sr(BIC)]+ complexes.


 Please wait while we load your content...
Please wait while we load your content...