VUV-photolysis of aqueous solutions of hydroxylamine and nitric oxide. Effect of organic matter: phenol†
Abstract
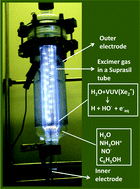
VUV-irradiation of aqueous solutions containing hydroxylamine (NH2OH) in its acid form (NH3OH+) and phenol (C6H5OH) results in the simultaneous mineralization of the organic substrate and the almost quantitative reduction of NH3OH+ to ammonium ions (NH4+). Irradiation of aqueous solutions of NH3OH+ in the absence of organic substrates showed the formation of nitrate (NO3−) and nitrite (NO2−) and minor quantities of NH4+. In line with these experiments, VUV-irradiation of aqueous solutions of nitrogen monoxide (NO˙) yields NH4+ only when C6H5OH is simultaneously mineralized. A possible reaction mechanism is discussed, where reactions of NO˙ and NH3OH+ with hydrogen atoms (H˙), hydroxyl radicals (HO˙) and hydrated electrons (e−aq), all generated by the VUV-photochemically initiated homolysis of water, are of great importance to the observed results. In the presence of phenol, competition between phenol and either NO˙ or NH3OH+ for these reactive intermediates in the primary volume of reactions strongly determines the oxidation state and nature of the N-containing products. C-Centered radicals and intermediate products of reactions may also have an important effect on the overall mechanism. The present results are discussed in relation to the actual state of knowledge presented in the literature.
- This article is part of the themed collection: In memory of Professor Ugo Mazzucato (1929–2017)


 Please wait while we load your content...
Please wait while we load your content...