Diphenylaminostyryl-substituted quinolizinium derivatives as fluorescent light-up probes for duplex and quadruplex DNA†
Abstract
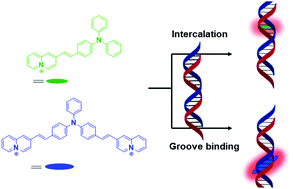
(E)-2-[1′-((Diphenylamino)styryl)quinolizinium (3a) and 2,2′-{(phenylimino)-bis[(E)-1′′,1′′′-styryl]}-bis[quinolizinium] (3b) were synthesized, and their interactions with duplex DNA and quadruplex DNA were investigated with a particular focus on their ability to operate as DNA-sensitive fluorescent probes. Due to the significantly different size and steric demand of these quinolizinium derivatives they exhibit different binding modes. Thus, 3a intercalates into duplex DNA and binds through π stacking to quadruplex DNA, whereas 3b favours groove binding to both DNA forms. The emission intensity of these compounds is very low in aqueous solution, but it increases drastically upon association with duplex DNA by a factor of 11 (3a) and >100 (3b) and with quadruplex DNA by a factor of >100 (3a) and 10 (3b), with emission bands between 600 and 750 nm.


 Please wait while we load your content...
Please wait while we load your content...