Effect of the size of polycyclic aryl groups on the competition between adiabatic/diabatic photoisomerization mechanisms of cis-styrylarenes†‡
Abstract
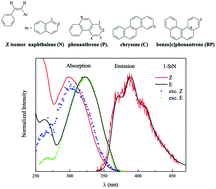
The occurrence of adiabatic photoisomerization in the singlet manifold directly from 1Z* to 1E* has been found to be more common than expected. This mechanism has been experimentally evidenced through a detailed fluorimetric study for a large series of styrylarenes. Its weight on the overall cis–trans photoisomerization has been determined and found to increase when increasing the size of the polycyclic chromophore.
- This article is part of the themed collection: In memory of Professor Ugo Mazzucato (1929–2017)


 Please wait while we load your content...
Please wait while we load your content...