Abstract
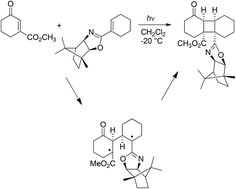
The photochemical reaction of 3-carboxymethylcyclohexenone with a cyclohexene bearing a chiral auxiliary group has been examined in a DFT study. GIAO simulation of the NMR spectra (DFT/B3LYP/6-311G++(d,2p) level of theory) is not in agreement with the syn–anti–syn product described in the literature for this reaction but is in agreement with the formation of a syn–cis–syn dimer. The analysis of the frontier orbitals involved in this reaction shows that the main interaction is that between the HOMO of the alkene and the LSOMO of the carbonyl compound in its first excited triplet state. The atomic coefficients do not allow a frontier orbital control of the regiochemistry of the reaction. The study of the possible biradical intermediates and the energies of the transition states involved agree with the formation of a syn–cis–syn biradical intermediate. The coupling of the radical carbon atoms in this biradical intermediate allows the obtainment of a more stable dimer. Furthermore, the coupling reaction occurs via a conrotatory process that is able to give only one of the possible syn–cis–syn dimers.
- This article is part of the themed collection: In memory of Professor Ugo Mazzucato (1929–2017)


 Please wait while we load your content...
Please wait while we load your content...