Formation of a supramolecular charge-transfer complex. Ultrafast excited state dynamics and quantum-chemical calculations
Abstract
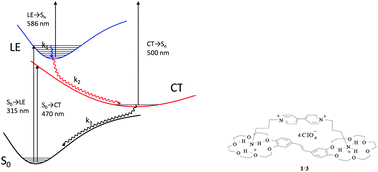
The formation of a supramolecular complex of bis(18-crown-6)stilbene (1) and 4,4′-bipyridine with two ammoniopropyl N-substituents (3) and the substitution reaction between 1·3 and alkali and alkaline-earth metal perchlorates have been studied using absorption, steady-state fluorescence, and femtosecond transient absorption spectroscopy. The formation of 1·(Mn+)2 complexes in acetonitrile was demonstrated. The weak long-wavelength charge-transfer absorption band of 1·3 completely vanishes upon complexation with metal cations because of disruption of the pseudocyclic structure. The spectroscopic and luminescence parameters, stability and substitution constants were calculated. The relaxation scheme of the 1·3 singlet state excited by a 25 fs laser pulse was proposed. It includes very fast vibrational relaxation and direct (τCT-d = 0.32 ps) and back (τCT-b = 0.51 ps) electron transfer resulting in complete fluorescence quenching. The quantum-chemistry calculations revealed the species taking part in the ET process and elucidated the mechanism of relaxation of the excited complex.


 Please wait while we load your content...
Please wait while we load your content...