Ultrafast dynamics of the antibiotic Rifampicin in solution†
Abstract
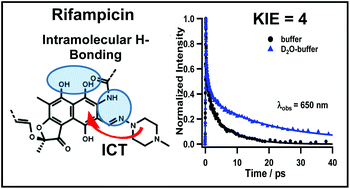
Rifampicin (Rif) is an effective antibiotic against mycobacterial infections and a wide range of Gram-positive and Gram-negative bacteria. The geometry, conformation and the intramolecular H-bond network of Rif can affect its antibacterial efficiency. In this work, we report on the excited-state dynamics of Rif in sodium phosphate buffer and dichloromethane solutions using femtosecond time-resolved spectroscopic methods. The femtosecond UV-Vis-nearIR transient absorption and fluorescence up-conversion experiments reveal an ultrafast (<100 fs) Franck–Condon relaxation with a partial charge transfer character in S1, and a short-lived emission (τ ∼ 6 ps) due to a non-radiative relaxation to the ground state, associated with the stretching of the vibrational mode of the Rif internal H-bond network. The large Stokes-shifted emission (∼6800 cm−1) indicates a significant electronic change in the excited-state. In deuterated potassium phosphate buffer, the decay time becomes longer (∼20 ps). The large kinetic isotope effect (KIE) of ∼4 on the decay rate indicates that the stretching modes of the internal H-bond network are slowed by the H/D isotope substitution. The results provide new information on the dynamics of Rif structures and the related processes in aqueous solutions, showing that the internal H-bonding interactions are the ones that govern the ground and excited state properties of Rif but water molecules exert additional stabilization of its zwitterionic form through intermolecular H-bonds, which is responsible for its high antimicrobial activity.


 Please wait while we load your content...
Please wait while we load your content...