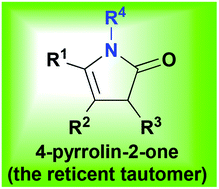
The reticent tautomer: exploiting the interesting multisite and multitype reactivity of 4-pyrrolin-2-ones
Abstract
4-Pyrrolin-2-ones are a lesser known tautomeric relative of the 3-pyrrolin-2-ones. Despite their infrequent appearance in the literature, they are very interesting and useful compounds. They have highly controllable, multisite and multitype reactivities which are covered in this review. The applications of these transformations show how the 4-pyrrolin-2-ones make excellent intermediates en-route to a range of key alkaloids. Innovative, fast and adaptable syntheses of the 4-pyrrolin-2-ones and their onward use via cascade reaction sequences are also presented to complete the case for commending these compounds as highly versatile and valuable synthetic building blocks.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...