Effect of n-alkyl substitution on Cu(ii)-selective chemosensing of rhodamine B derivatives†
Abstract
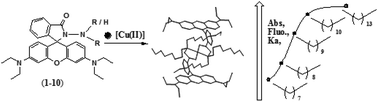
Rhodamine B hydrazide-based molecular probes (1–10) were synthesized by derivatization with n-alkyl chains of different lengths at the hydrazide amino end. These probes exhibited selective absorption (A∼557) and fluorescence (I∼580) ‘off–on’ signal transduction along with a colourless → magenta colour transition in the presence of Cu(II) ions among all the competitive metal ions investigated. The effective coordination of these probes to Cu(II) ions under the investigated environment forming [Cu·L]2+ (L = 1–5) and [Cu·L2]2+ (L = 6–10) complexes led to their spiro-ring opening, which in turn was expressed through signatory spectral peaks of ring-opened rhodamine. All these probes exhibited Cu(II) selectivity in signalling despite structural modifications to the core receptor unit through variation of the nature of the alkyl substituents. However, the sensitivity of the signalling and kinetics of the spiro-ring opening varied and could be correlated with the number of carbon atoms present in the n-alkyl substituents. Structural elucidation with X-ray diffraction and X-ray photoemission spectroscopic analyses provided further insight into the structure–function correlation in their Cu(II) complexes. These probes with Cu(II) coordination showed selectivity in signalling, high complexation affinity (log Ka = 4.8–8.8), high sensitivity (LOD = 4.1–80 nM), fast response time (rate = 0.0017–0.0159 s−1) and reversibility with counter anions, which ascertained their potential utility as chemosensors for Cu(II) ion detection.


 Please wait while we load your content...
Please wait while we load your content...