BF3·OEt2-promoted tandem Meinwald rearrangement and nucleophilic substitution of oxiranecarbonitriles†
Abstract
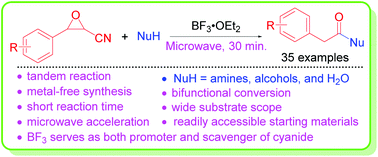
Tandem Meinwald rearrangement and nucleophilic substitution of oxiranenitriles was realized. Arylacetic acid derivatives were readily synthesized from 3-aryloxirane-2-carbonitriles with amines, alcohols, or water in the presence of boron trifluoride under microwave irradiation, and the designed synthetic strategy includes introducing a cyano leaving group into arylepoxides and capturing the in situ generated toxic cyanide with boron trifluoride, making the reaction efficient, safe, and environmentally benign. The reaction occurs through an acid-promoted Meinwald rearrangement, producing arylacetyl cyanides, followed by an addition–elimination process with nitrogen or oxygen-containing nucleophilic amines, alcohols or water. The current method provides a new application of the tandem Meinwald rearrangement.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...