Branched lipid chains to prepare cationic amphiphiles producing hexagonal aggregates: supramolecular behavior and application to gene delivery†
Abstract
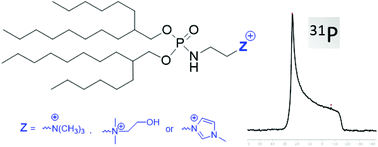
A ramified lipid alcohol, 2-hexyldecanol, was used as a hydrophobic moiety to prepare cationic amphiphiles on a gram scale in 3 to 4 steps, featuring either a trimethylammonium 5, dimethylhydroxyethylammonium 6 or N-methylimidazolium 7 polar head group. Compression isotherms at the air–water interface reveal that all these cationic amphiphiles collapse at a relatively low pressure indicating a weak stabilization of the monolayer via hydrophobic interactions. Ellipsometry measurements point out the presence of a thin monolayer at low lateral pressure whereas thickening of the monolayer occurs at higher pressure with a high percentage of variation of the thickness, thus demonstrating an adaptability to the constraints. 31P NMR spectroscopy of the hydrated cationic amphiphiles clearly shows that these cationic amphiphiles self-assemble in water to form hexagonal phases, irrespective of the nature of their polar head group. Furthermore, a comparison of molecular structures suggests that compounds 5–7 self-organize into an inverted hexagonal phase (HII). These cationic amphiphiles, alone or in the presence of DOPE, were evaluated for the transfection of three human-derived cell lines (i.e. A549, 16HBE and HeLa). The three compounds demonstrated high transfection efficacies in every cell line tested, 7/DOPE being the most efficient.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...