Base-mediated intramolecular one-pot double-cyclization of epoxide-tethered 2-fluorobenzenesulfonamides: an avenue to 1,4-benzoxazine-fused benzothiaoxazepine-1,1-dioxides†
Abstract
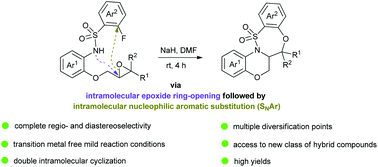
Herein, we describe the synthesis of hitherto unknown 1,4-benzoxazine-fused benzothiaoxazepine-1,1-dioxides by a NaH-promoted intramolecular one-pot double-cyclization of epoxide-tethered 2-fluorobenzene sulfonamides. Mechanistically, the reactions proceed via an intramolecular epoxide ring-opening followed by an intramolecular nucleophilic aromatic substitution. The high yields, mild conditions, complete regio- and diastereoselectivity, and a wide substrate scope render this protocol well suited for drug discovery efforts.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...