Diastereoselective construction of 3-aryl-substituted indolines via annulation of in situ generated p-quinone methides†
Abstract
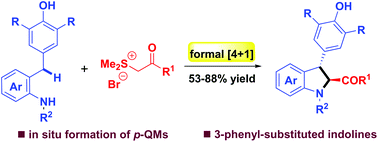
A highly diastereoselective [4 + 1] annulation reaction of in situ generated p-quinone methides for the synthesis of 3-aryl-substituted indolines has been developed. Employing commercial manganese dioxide as the oxidant, a series of ortho-tosylaminophenyl-substituted p-QMs could be generated in situ. This new protocol is based on an unprecedented 1,6-conjugate addition/annulation cascade reaction, without the need for pre-synthesized p-QMs, and enables the easy preparation of a variety of 3-aryl-2,3-dihydroindoles in good to excellent yields.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...