Organocatalytic enantioselective aminoalkylation of pyrazol-3-ones with aldimines generated in situ from α-amido sulfones†
Abstract
Herein, an efficient asymmetric aminoalkylation of pyrazolones with α-amido sulfones catalyzed by a quinine-derived squaramide in dichloromethane/aqueous media has been established. A variety of chiral amines were obtained with high yields (up to 98%) and excellent enantioselectivities (up to 99% ee). The corresponding products are transformed into optically active acetylated pyrazoles after treatment with Ac2O/Et3N, because of the instability of some adducts. The reaction tolerates a wide range of α-amido sulfones and different pyrazolones.
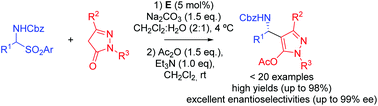
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...