N-Isopropylsulfinylimines vs. N-tert-butylsulfinylimines in the stereoselective synthesis of sterically hindered amines: an improved synthesis of enantiopure (R)- and (S)-rimantadine and the trifluoromethylated analogues†
Abstract
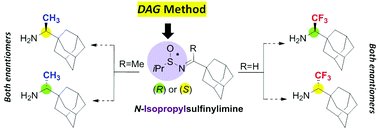
An improved fully stereoselective synthesis of both enantiomers of rimantadine and its trifluoromethylated analogues has been developed, using N-isopropylsulfinylimines as a starting chiral material, proving the superiority of the isopropyl group as a chiral inducer over the tert-butyl group in the case of hindered N-sulfinylimines.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...