An atom-economical and regioselective metal-free C-5 chalcogenation of 8-aminoquinolines under mild conditions†
Abstract
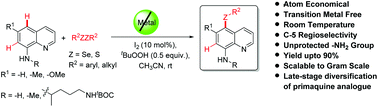
A general and simple metal-free protocol for expedient C–H functionalization leading to the regioselective generation of C-5 chalcogenated 8-aminoquinoline analogues in up to 90% yield at room temperature (25 °C) has been established. This methodology is an eco-friendly approach to the atom-economical utilization of diaryl/dialkyl chalcogenides for direct access to chalcogenated quinolines and is scalable to the gram scale without considerable decrease in the yield of the product. It represents a practical alternative to the existing metal-catalyzed functionalization of 8-aminoquinoline derivatives with broad functional group tolerance. The controlled experiments suggest that the reaction possibly proceeds through an ionic pathway at room temperature. Furthermore, the potentiality for the functionalization of free amines in chalcogenated-8-aminoquinolines provides an attractive perspective for further elaboration of the amine substituent through chemical manipulations. The applicability of the standardized method has been augmented through late-stage antimalarial drug diversification of primaquine analogues.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...