Lewis or Brønsted acid-catalysed reaction of propargylic alcohol-tethered alkylidenecyclopropanes with indoles and pyrroles for the preparation of polycyclic compounds tethered with indole or pyrrole motif†
Abstract
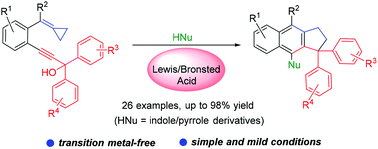
We developed a facile synthetic method to access cyclopenta[b]naphthalene derivatives via the Lewis or Brønsted acid catalysed cascade nucleophilic addition, electronic cyclization, ring-opening rearrangement of propargylic alcohol-tethered alkylidenecyclopropanes with indole and pyrrole derivatives. The reaction exhibited a broad substrate scope and good functional group tolerance under metal-free conditions, affording the desired products in moderate to good yields.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...