Theoretical calculation studies on the rearrangement mechanisms of arenesulfenanilides to generate o- and p-aminodiphenyl sulfides†
Abstract
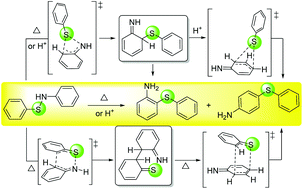
The thermal and acid-catalyzed rearrangement mechanisms of arenesulfenanilides were investigated theoretically via density functional theory (DFT) calculations. The results indicate that the o-aminodiphenyl sulfide rearrangement involves a novel S[1,3]-sigmatropic shift followed by tautomerization, while the p-aminodiphenyl sulfide rearrangement proceeds via tandem [3,3]- and [3,3]-sigmatropic shifts followed by tautomerization under thermal conditions. Furthermore, computational studies reveal that water assists the proton shift more efficiently than anilines during tautomerization. Moreover, under the acid-catalyzed conditions, the o-aminodiphenyl sulfide rearrangement involves an S[1,3]-sigmatropic shift similar to that under the thermal conditions, while the p-aminodiphenyl sulfide rearrangement proceeds via cascade S[1,3]- and S[1,3]-sigmatropic shifts followed by water-aided tautomerization. The current theoretical studies provide new insights into the formation mechanism of o/p-aminodiphenyl sulfides in the arenesulfenanilide rearrangement and support the unprecedented suprafacial symmetry-allowed S[1,3]-sigmatropic shift with an inversion of the configuration in the migrating sulfur atom. The mechanism is affected by the reaction medium. Disproportionation of arenesulfenanilides into diaryl disulfides and azobenzenes is a competitive radical pathway during the arenesulfenanilide rearrangements.


 Please wait while we load your content...
Please wait while we load your content...