Metal free biomimetic deaminative direct C–C coupling of unprotected primary amines with active methylene compounds†
Abstract
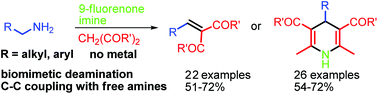
An unprecedented direct C–C coupling reaction of unprotected primary amines with active methylene compounds is reported. The reaction involves a biomimetic deamination of amines which was achieved under conditions free of metallic reagents and strong oxidizing agents. A wide range of primary amines was reacted with different active methylene compounds to provide structurally diverse trisubstituted alkenes and dihydropyridines. A kinetic study revealed an activation barrier of 10.1 kcal mol−1 for the conversion of a key intermediate of the reaction.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...