Electronic effects in tautomeric equilibria: the case of chiral imines from d-glucamine and 2-hydroxyacetophenones†
Abstract
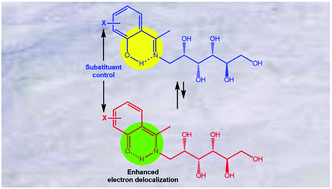
A one-pot procedure for preparing a series of chiral imines by direct condensation of D-glucamine with 2-hydroxyacetophenones is described. Under conventional acetylation an unexpected mixture of two different peracetylated molecules is obtained, one with an open enamine structure, and the other incorporating an N-acetyl-1,3-oxazolidine into the acyclic skeleton. Surprisingly, both molecules coexist within the crystal's unit cell, as inferred from single-crystal X-ray analysis of a 5-bromo-substituted aryl derivative. Moreover, the 1,3-oxazolidine ring exists as rotational conformers (E,Z) owing to the restricted rotation around the N-acetyl bond. The equilibrium involving imine and enamine structures has been assessed in detail, providing in addition linear free-energy relationships between the tautomerization constants (KT) and the electronic effect of the substituents.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...