Total chemical synthesis of murine ISG15 and an activity-based probe with physiological binding properties†
Abstract
The linear synthesis of the N-terminal domain of mISG15 has been developed which enables the synthesis of full-length mISG15 and the activity-based probe Rho-mISG15-PA via native chemical ligation. Pilot experiments showed that the synthetic proteins were properly folded and recognized by endogenous enzymes. Our synthesis strategy allows the generation of other mISG15-based probes and reagents that can accelerate the research in this field.
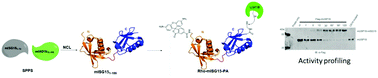
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...