Gold(i)-catalyzed nucleophilic cyclization of β-monosubstituted o-(alkynyl)styrenes: a combined experimental and computational study†
Abstract
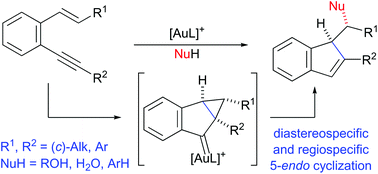
The stereospecific gold(I)-catalyzed nucleophilic cyclization of β-monosubstituted o-(alkynyl)styrenes to produce C-1 functionalized 1H-indenes including challenging substrates and nucleophiles, such as β-(cyclo)alkyl-substituted o-(alkynyl)styrenes and a variety of alcohols as well as selected electron-rich aromatics, is reported. DFT calculations support the stereochemical outcome of the process that involves the formation of a key cyclopropyl gold carbene intermediate through a regiospecific 5-endo cyclization.
- This article is part of the themed collections: Synthetic methodology in OBC and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...