An efficient synthesis of benzothiazole using tetrabromomethane as a halogen bond donor catalyst†
Abstract
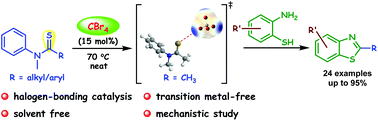
An efficient and mild protocol has been developed for the synthesis of 2-substituted benzothiazole under solvent- and metal-free conditions using CBr4 as the catalyst. This process involves the activation of a thioamide through halogen bond formation between the sulphur atom of the thioamide and bromine atom of the CBr4 molecule. The presence of halogen-bonding interaction between N-methylthioamides and tetrabromomethane has been demonstrated with several control experiments, spectroscopic analysis and density functional theory (DFT). This methodology has a wide substrate scope for the synthesis of both 2-alkyl and 2-aryl substituted benzothiazoles.
- This article is part of the themed collections: Synthetic methodology in OBC and Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...