Profiling the oxidative activation of DMSO-F6 by pulse radiolysis and translational potential for radical C–H trifluoromethylation†
Abstract
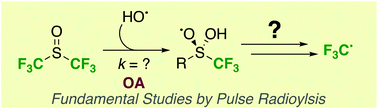
The oxidative activation of the perfluorinated analogue of dimethyl sulfoxide, DMSO-F6, by hydroxyl radicals efficiently produces trifluoromethyl radicals based on pulse radiolysis, laboratory scale experiments, and comparison of rates of reaction for analogous radical systems. In comparison to commercially available precursors, DMSO-F6 proved to be more stable, easier to handle and overall more convenient than leading F3C-reagents and may therefore be an ideal surrogate to study F3C radicals for time-resolved kinetics studies. In addition, we present an improved protocol for the preparation of this largely unexplored reagent.


 Please wait while we load your content...
Please wait while we load your content...