A synthetic route to 1,4-disubstituted tetrahydro-β-carbolines and tetrahydropyranoindoles via ring-opening/Pictet–Spengler reaction of aziridines and epoxides with indoles/aldehydes†
Abstract
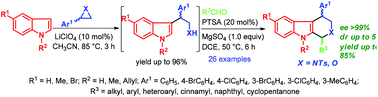
A simple and efficient synthetic route to various 1,4-disubstituted tetrahydro-β-carbolines and tetrahydropyrano[3,4-b]indoles in high yields and stereoselectivity via LiClO4-catalyzed SN2-type ring opening of aziridines and epoxides with indoles followed by p-toluenesulfonic acid (PTSA) catalyzed Pictet–Spengler reaction is described.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...