3-Nitro-3,4-dihydrocoumarins: valuable precursors for the synthesis of enantiomerically enriched masked quaternary α-amino acid derivatives with a 3,4-dihydrocoumarin scaffold†
Abstract
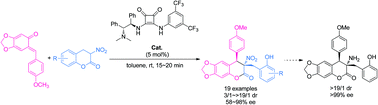
A bifunctional squaramide catalyzed enantioselective formal [2 + 4] annulation reaction with 3-nitro-3,4-dihydrocoumarins and ortho-quinone methide has been developed. Novel chiral masked quaternary α-amino acid derivatives with a 3,4-dihydrocoumarin scaffold are obtained in a highly stereocontrolled manner. Representative transformation of the annulation product to a biologically important quaternary α-amino acid derivative is achieved without any appreciable loss in the diastereo- and enantioselectivity.
- This article is part of the themed collections: Synthetic methodology in OBC and Catalysis & biocatalysis in OBC


 Please wait while we load your content...
Please wait while we load your content...