Synthesis and glycosidase inhibition of N-substituted derivatives of 1,4-dideoxy-1,4-imino-d-mannitol (DIM)†
Abstract
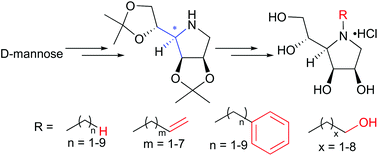
N-Substituted derivatives of 1,4-dideoxy-1,4-imino-D-mannitol (DIM), the pyrrolidine core of swainsonine, have been synthesized efficiently and stereoselectively from D-mannose with 2,3:5,6-di-O-isopropylidene DIM (10) as a key intermediate. These N-substituted derivatives include N-alkylated, N-alkenylated, N-hydroxyalkylated and N-aralkylated DIMs with the carbon number of the alkyl chain ranging from one to nine. The obtained 33 N-substituted DIM derivatives were assayed against various glycosidases, which allowed a systematic evaluation of their glycosidase inhibition profiles. Though N-substitution of DIM decreased their α-mannosidase inhibitory activities, some of the derivatives showed significant inhibition of other glycosidases.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...